Fast equilibration mechanisms in disordered materials mediated by slow liquid dynamics
Science Advances (2022) in the NEWS
Slow and steady wins the race
A system is at equilibrium when its properties do not change with time. Experience tells us that such observation is, however, rarely encountered in nature. The transformation of buds into flowers and then fruits, the rearrangements of plates on the surface of planets, and even the whole human body over its lifetime are just a few, among the many, examples of systems far from equilibrium. With time, these systems struggle to reach equilibrium, meaning they rearrange and adapt to the environment to reach reduce their internal energy.
Since almost a century, we know that macroscopic phenomena of equilibration (e.g. an elastic rope that elongates upon traction, or an ice cube that melts when we bring it out of the freezer) require microscopic motion of molecules. By increasing the temperature molecules move faster, and equilibration is achieved within a shorter time. This fundamental principle reflects the beauty of physics and has powerful implications.
By observing how a material reacts to the application of small forces, we can follow the equilibration process and, hence, understand the trajectories of molecules, regardless how fast molecules move and how small are the distances they make.
Thanks to these experimental methods it was possible to observe that molecules of liquids need to cooperate to move around their positions. Molecular motion is achieved only upon a sort of collaborative action. The colder the temperature gets, the more the liquid becomes dense and viscous, and the more molecules need to coordinate their movements to equilibrate.
This is similar to what happens while we drive. If the road is empty (high temperature), we can move as fast as we want, but during a traffic jam (low temperature) we get stuck until the cars around us work together and give us some space. For decades the dynamics of liquids has been described by considering only this kind of motion, known as structural mobility.
Our laboratory has shown that molecules also know another way to move. We identified a new molecular process, called SAP (slow Arrhenius process). At high temperatures the SAP is slower than structural mobility and shows the characteristics of an “Arrhenius process”, that is, its rearrangements are not affected by density.
Our experimental work replies to several unanswered questions of the dynamics of liquids. Laboratories all over the world had already observed that liquids can equilibrate in an efficient way which cannot be attributed to the structural process; we verified that the molecules of different types of materials choose the SAP to reduce their internal energy. Importantly, following the route of the SAP is possible at any temperature: while the structural process becomes slower and slower upon cooling, at low temperatures, when the liquid is getting so viscous that it behaves almost as a solid, the new mechanism overtakes the old one. Slow and steady wins the race! Thanks to its unique properties the SAP can ease the equilibration of materials within a reasonable time (days, months), at temperatures where the structural process would require geological (infinite) times.
The SAP can be pictured as a team of cyclists delivering food: they might be slower than cars during regular hours, but in the case of traffic jam, you can count on them to get a warm meal on your table.
Understanding the nature of the SAP has strong implications. In the design of new materials and their fabrication protocols, a better control of properties is achieved by identifying those conditions favoring mechanisms that do not depend on a change in the structure, as it’s the case for the SAP. Moreover, as most raw and processed amorphous materials are stored in the low temperatures, the shelf time of these systems is significantly affected by potential equilibration through the new pathway discovered by our team.
Direct observation of desorption of a melt of long polymer chains
Nature Communications (2020) in the NEWS
L’union fait la force – Eendracht maakt macht
In our everyday life it’s not uncommon to see the same material in different states. Take for example water: it’s a liquid at ambient temperature, we can convert into ice when cooled below 0°C and it becomes a gas when heated above 100°C. The passages between these different states of matter are called phase transitions.
Phase transitions are the expression of the organization and interactions of molecules and atoms inside materials, and because of this they have been largely studied by physicists, chemists, biologists, and many more.
Some phase transitions, though predicted by theory, remain elusive and their existence cannot be verified with experiments, because of the harsh conditions in which they occur. This is the case of the adsorption/desorption transition of polymers.
Polymers are long molecules made up by the repetition—often more than thousand times—of the same unit, called monomer. This particular structure brings up a series of interesting properties. For example, a polymer molecule can strongly adhere onto a surface even if the interaction between one single monomer and the surface is very weak: l’union fait la force. In fact, in order to separate the whole molecule from the surface, one would have to remove one by one all the monomers that are weakly attached, which is very unlikely to occur. Polymer chains are those considered irreversibly adsorbed, that is, a polymer chain is supposed to stick on a surface, for an extremely long time, basically forever.
Theorists have proposed that the adsorbed state is, instead, transitory and, when heated well above room temperature, polymer molecules should desorb and leave the surface. This would be the adsorption/desorption transition.
Till now, however, no one could verify these ideas, because the temperatures where this phase transition should occur are very high, and the material degrades before, eventually, desorbing.
Together with our collaborators, Xavier Monnier and Daniele Cangialosi, from the Donostia International Physics Center and Centro de Física de Materiales of San Sebastián (Spain) we have been able to experimentally access the adsorption/desorption transition.
Combining the expertise of Cangialosi in phase transitions and that of SSN on adsorption, we have used a new technique called fast scanning calorimetry, that permits to measure the heat exchanged by a material while the temperature is varied very rapidly. The technique can bring the polymer molecules from room temperature to 400°C within a fraction of second, and within this short interval the material does not have time to degrade.
By studying this phenomenon, we have observed that a very tiny quantity of heat is released from the polymer chains when they desorb from a surface, which permitted to classify the adsorption/desorption as a first order phase transition.
 This is similar to what happens to ice when we put it on the table. At low temperature, the molecules stay together thanks to interactions which keep the material in the solid state. By heating above 0°C the interactions start to fade, which corresponds to a heat exchange. The same occurs to polymer chains when they desorb.
This is similar to what happens to ice when we put it on the table. At low temperature, the molecules stay together thanks to interactions which keep the material in the solid state. By heating above 0°C the interactions start to fade, which corresponds to a heat exchange. The same occurs to polymer chains when they desorb.
In addition to the tremendous advancement of the study of phase transitions, this study opens to new methods to tailor the properties of nanomaterials as smart coatings, flexible electronics and more. The properties of these innovative systems, in fact, depend on how many molecules are adsorbed, and the authors anticipate that by adequately mastering the adsorption/desorption transition it will be possible to fabricate better performing and more durable materials.
Taming the Strength of Interfacial Interactions via Nanoconfinement
ACS Central Science (2018) in the NEWS
Size Matters in Nanoconfinement
Can we predict if two materials will stick together? Theory tells us that the more contacts are formed among molecules of the two materials placed one next to the other, the stronger will be their adhesion. Unfortunately, counting the number of contacts between two materials is a difficult task. To overcome this obstacle, it is possible to virtually reconstruct the interface between two materials and, using approximate equations, to estimate the force keeping them together. This procedure, currently used at both industrial and academic level, seems to work very well when the materials used are in the macroscale.

The outcome is not as good upon reduction of material dimension to the nanoscale (1 nm is one billionth of a meter, one single of your hairs can contain from 20000 to 200000 nm!).The origin of this discrepancy is because of a set of forces, known as van der Waals (vdW) forces, that depend on the dimension of the objects involves. These forces are extremely important, also in everyday life. vdW forces are for example those that permit the transparent packaging foil to stick to food and protect it from air, or also those allowing geckos to walk on flat walls as if they had dipped their tiny paws into glue.
Differently than covalent forces, responsible for strong chemical bonds, vdW forces take place over much larger distances, up to few hundreds of nanometers. Due to such a large length scale, vdW forces are affected by nanoconfinement, that is, their intensity changes if the size of the objects fit in boxes of nanometric dimension.
In this paper we show that it is possible to estimate how nanoconfinement affects the number of contacts formed by two materials placed in intimate contact. They considered wafers of silicon, as those largely used in microelectronics, coated by thin polymer layers of different thickness. The currently used approximate methods predict that the interaction between the two materials does not depend on the thickness of the polymer layer. On the contrary our team showed that size does matter. Molecules at the interface of thinner films form less contacts with the silicon wafer, because the vdW forces are weaker.
The method used permitted to verify a striking correlation between the intensity of the vdW forces and the number of contacts. This result shows that the current way we think at interfaces is not valid. In addition to the huge impact at the level of fundamental science, our results could be exploited on a large number of applications. Since almost a decade, several research groups have shown that properties of many thin coatings – such as flow, the ability to retain or be repel water, the velocity of formation of crystals – depend on the number of contacts between the film and its supporting substrate. Till now, to modify this number it was necessary to change the type of molecules at the interface, often involving
complex chemical reactions.
Our findings of Simavilla show that it is possible to tailor the performance of nanomaterials by simply changing their dimensions. Or even without! Placing a different material on top of the polymer layer in contact with the substrate, affects in a controllable way the vdW forces at the interface between polymer of given thickness and the substrate. This method, hence, allows controlling the polymer layer without touching it, as by using a remote control.
Irreversible Adsorption Governs the Equilibration of Thin Polymer Films
Phys. Rev. Lett. (2017) in the NEWS
Confined but not forever
Molecules move faster as they get closer to adhesive surfaces, but this effect is not permanent. Since more than 20 years, several researchers have been studying the behavior of certain polymers, biomolecules, and liquid crystals at the nanoscale near an absorbing medium. In this case we would expect slower movement rates, but the experiments showed the opposite: molecules move faster as they get closer to an adhesive surface. We explain this odd movement via a
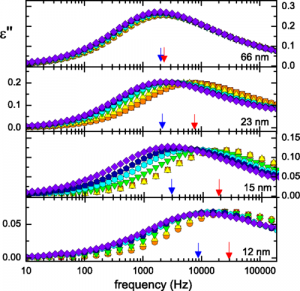 ‘nanoconfinement effect’: the molecules that are in direct contact with the adhesive surface do move slower, or even not at all, but this in turn increases the movement rate of the next molecules, as they have more free space around them.
‘nanoconfinement effect’: the molecules that are in direct contact with the adhesive surface do move slower, or even not at all, but this in turn increases the movement rate of the next molecules, as they have more free space around them.
In this paper, we show that this effect is only temporary: movement ra
te gradually slows down as new molecules adhere to the surface and fill in the spaces left. After a while, molecules move as if they were far from the adhesive surface. Importantly, the time necessary to return to normal molecular movement rate is longer than what would be predicted by any current theory of polymer physics. As a result, we propose that the amount of available space at the interface between polymer and sticky wall is an important parameter to control the performance of nanomaterials.
Characterization of Adsorbed Polymer Layers: Preparation, Determination of the Adsorbed Amount and Investigation of the Kinetics of Irreversible Adsorption
David Nieto Simavilla et al
Macromolecular Chemistry and Physics (2017)
Understanding adsorbed layers
The increasing number of devices containing soft materials components of nanometric size –as for example for applications in flexible electronics, biomedical and tissue engineering – has focused the interest of researches towards thin polymer films and their interaction with solid substrates.
A remarkable finding is that, the properties of polymer/substrate interfaces differ greatly from those predicted from the bulk properties of each material. Here, we deal with those deviations from bulk behavior induced by the formation of layer of polymer molecules, as thin as a few nanometers, which irreversibly stick on a solid substrate. 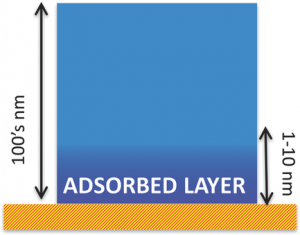 Understanding the physics behind the formation of these “irreversibly adsorbed layers” is necessary to control a large number of material properties: from the friction among molecules (viscosity) to the way such thin coatings expand with temperature, and also to the amount of water these membranes can uptake, or the way molecules can selforganize and form crystals. This Trend paper provides an introduction to: 1) The preparation of systems where irreversible adsorption can take place; 2) The modeling describing the kinetics of formation of the sticky layer; 3) The most common methods to determine their thickness and 4) A brief perspective on future applications and research outlooks derived from the extraordinary properties of these promising nanomaterials.
Understanding the physics behind the formation of these “irreversibly adsorbed layers” is necessary to control a large number of material properties: from the friction among molecules (viscosity) to the way such thin coatings expand with temperature, and also to the amount of water these membranes can uptake, or the way molecules can selforganize and form crystals. This Trend paper provides an introduction to: 1) The preparation of systems where irreversible adsorption can take place; 2) The modeling describing the kinetics of formation of the sticky layer; 3) The most common methods to determine their thickness and 4) A brief perspective on future applications and research outlooks derived from the extraordinary properties of these promising nanomaterials.